FP7
The OpenTox framework provides tools for the integration of data
from various sources (public and confidential), for the generation and
validation of (Q)SAR models for toxic effects, libraries for the
development and seamless integration of new (Q)SAR algorithms, and
scientifically sound validation routines. OpenTox is relevent for users
from a variety of research areas:
• Toxicological and chemical experts (e.g. risk assessors, drug designers, researchers)
• (Q)SAR model developers and algorithm developers
• Non-(Q)SAR specialists requiring access to Predictive Toxicology models and data
The OpenTox project moves beyond existing attempts to solve individual research issues within this area, by providing a flexible, extensible, and user friendly framework that integrates existing solutions as well as providing easy access to new developments.
OpenTox is relevant for the implementation of REACH
as it allows regulatory and industrial risk assessors to access
experimental data, (Q)SAR models and toxicological information from a
unified interface, that adheres to European and international
regulatory requirements (e.g. OECD Guidelines for (Q)SAR validation,
QSAR Model Reporting Formats (QMRF)). For maximum transparency
OpenTox is published as an open so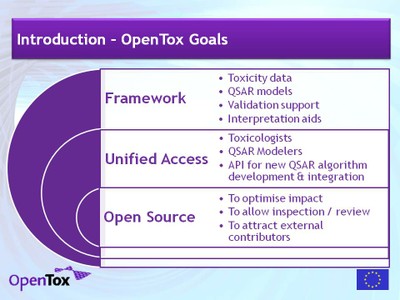 urce
project. This allows a critical evaluation of the implemented
algorithms, ensure a widespread dissemination and supporting external
developers. Facilities for the inclusion of confidential in-house data
and for accessing and integrating commercial prediction systems will be
included as the project develops.
urce
project. This allows a critical evaluation of the implemented
algorithms, ensure a widespread dissemination and supporting external
developers. Facilities for the inclusion of confidential in-house data
and for accessing and integrating commercial prediction systems will be
included as the project develops.
The OpenTox framework is being populated initially with high-quality data and (Q)SAR models for chronic, genotoxic and carcinogenic effects. These are the endpoints, where computational methods promise the greatest potential reduction in animal testing, that would be required for the implementation of REACH. The impact of OpenTox will however go beyond REACH, industrial chemicals and long-term effects, because reliable toxicity estimates are also needed for other products (e.g., pharmaceuticals, cosmetics, food-additives) and endpoints (e.g,. sensitisation, liver-toxicity, cardio-toxicity).
The OpenTox framework actively supports the development of new (Q)SAR models by automating routine tasks, providing a testing and validation environment and allowing the easy addition of new data. It also supports the development of new algorithms and to avoiding duplicated work by providing easy access to common components, validation routines and an easy comparison with benchmark techniques. For this reason we expect, that OpenTox will lead to (Q)SAR models for further toxic endpoints and generally improve the acceptance and reliability of (Q)SAR models.
Advisory Board
European Center for the Validation of Alternative Methods, European Chemicals Bureau, U.S Environmental Protection Agency, U.S. Food & Drug Administration, Nestle, Roche, AstraZeneca, LHASA, University North Carolina, EC Environment Directorate General, Organisation for Economic Co-operation & Development, CADASTER and Bayer Healthcare
OpenTox - An Open Source Predictive Toxicology Framework, is funded under the EU Seventh Framework Program: HEALTH-2007-1.3-3 Promotion, development, validation, acceptance and implementation of QSARs (Quantitative Structure-Activity Relationships) for toxicology, Project Reference Number Health-F5-2008-200787 (2008-2011).
For further information on OpenTox programs or to discuss
opportunities and potential for collaboration or your requirements as a
user, please contact the project coordinator Dr. Barry Hardy at:
barry.hardy [at] douglasconnect.com, Tel: +41 61 851 0170.


